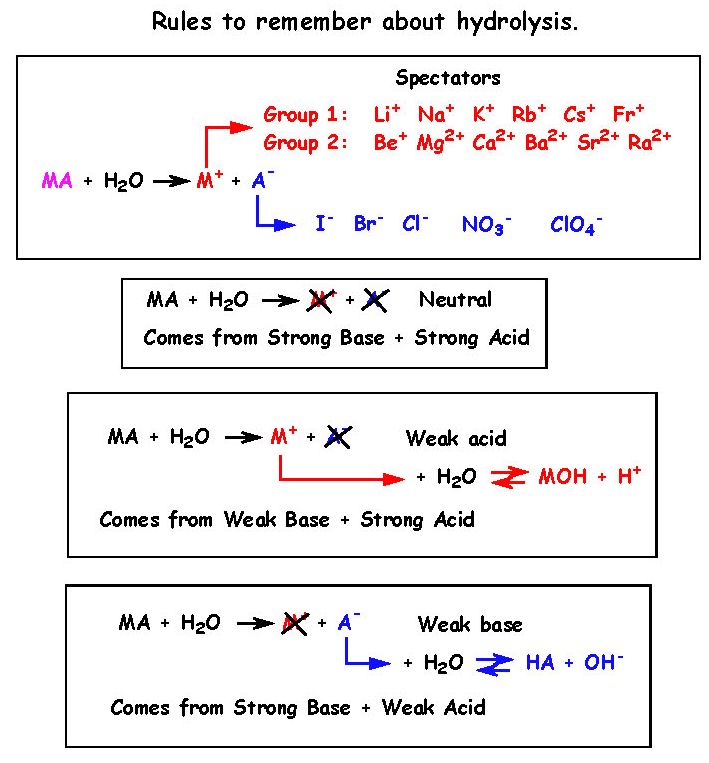
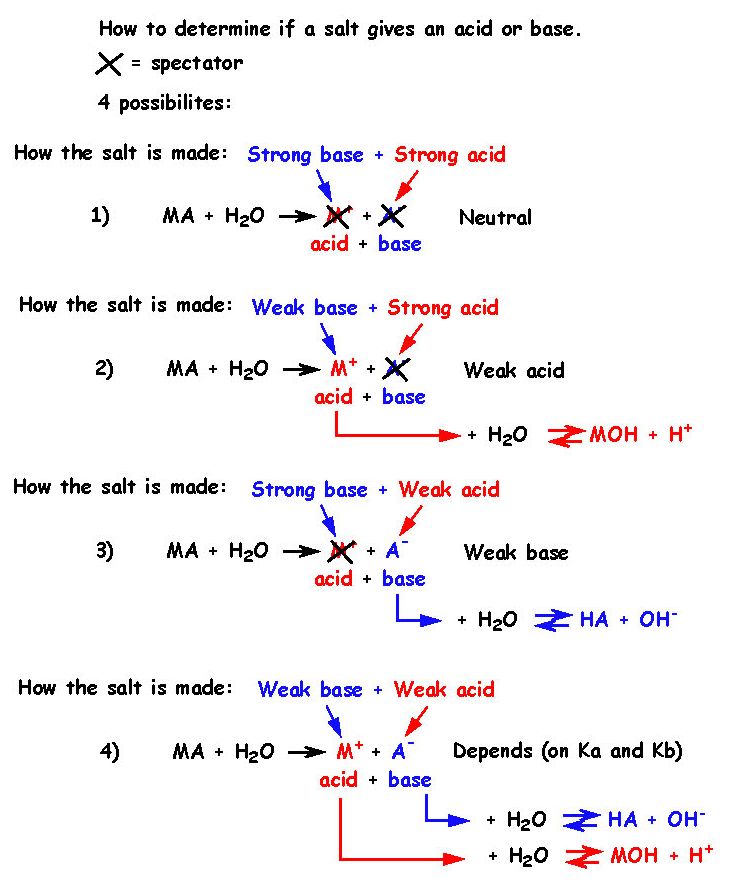
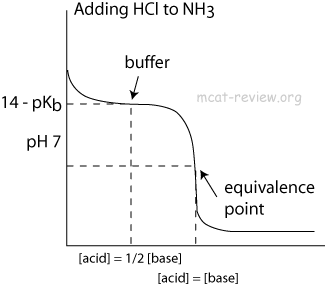
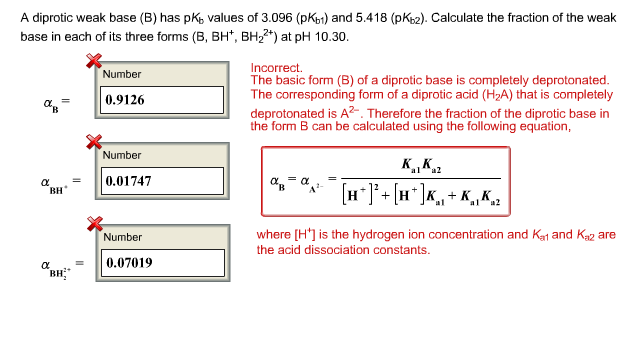
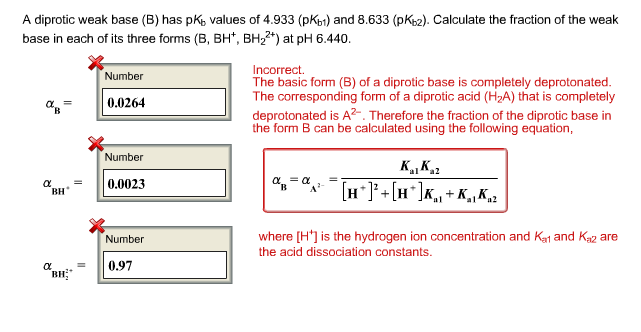
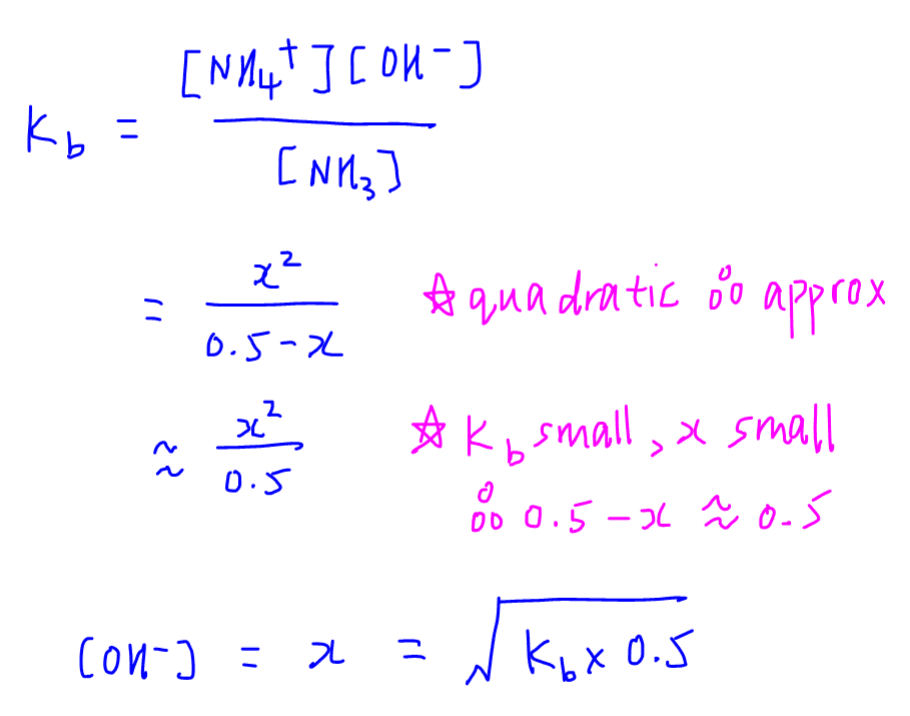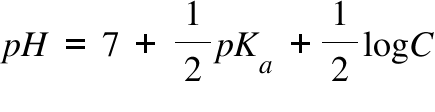Calculate pH of a salt of weak monobasic acid and weak monoacidic base having concentration 0.1 M at 25^oC (Given : - pka = 4.8 pkb = 5.2 )
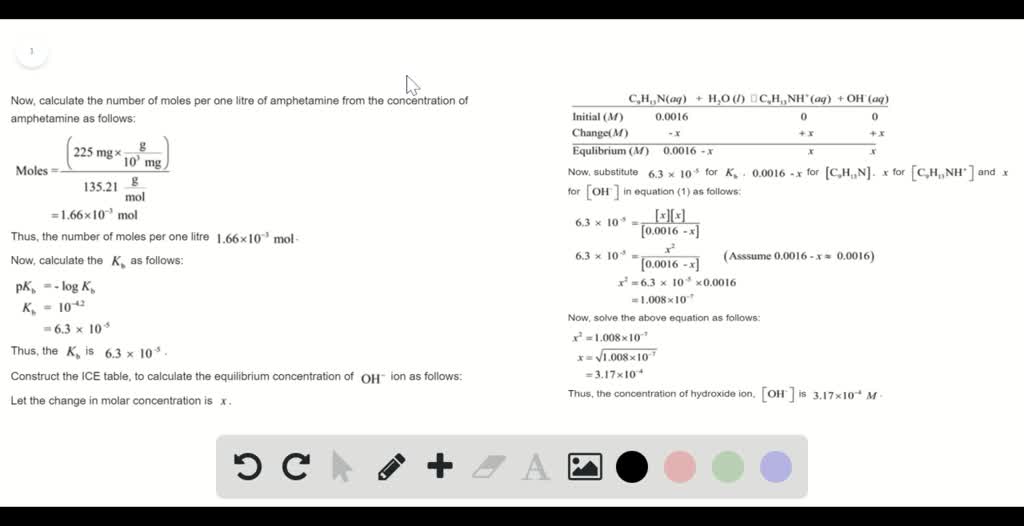
SOLVED: Amphetamine (C9H13N) is a weak base with a pKb of 4.2. Calculate the pH of a solution containing an amphetamine concentration of 225 mg>L.
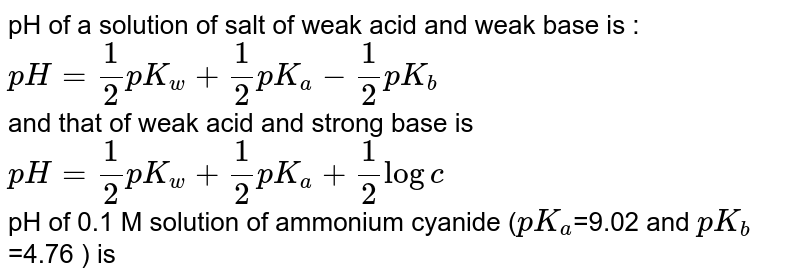
pH of a solution of salt of weak acid and weak base is : pH=1/2pKw+1/2pKa- pKb and that of weak acid and strong base is pH=1/2pKw+1/2pKa+1/2logc The pH of 0.1 M sodium acetate (
![OneClass: A weak base (B) has a pKb value of 5.77. a) At what pH is [BH ] = [B]? b) What is the predo... OneClass: A weak base (B) has a pKb value of 5.77. a) At what pH is [BH ] = [B]? b) What is the predo...](https://prealliance-textbook-qa.oneclass.com/qa_images/homework_help/question/qa_images/119/11951925.png)
OneClass: A weak base (B) has a pKb value of 5.77. a) At what pH is [BH ] = [B]? b) What is the predo...
PPT - Weak Acids & Bases: A weak acid is not completely dissociated:- HA H + + A - PowerPoint Presentation - ID:3428717